- Home
- Project Description
- Next Generation Antibody Manufacturing
We’ve been working on the "Project Focused on Developing Key Technology for Discovering and Manufacturing Drugs for Next-Generation Treatment and Diagnosis"(Next-generation biologics manufacturing conforming to global standards) implemented by the Japan Agency for Medical Research and Development( AMED) from FY2021, as following subjects.
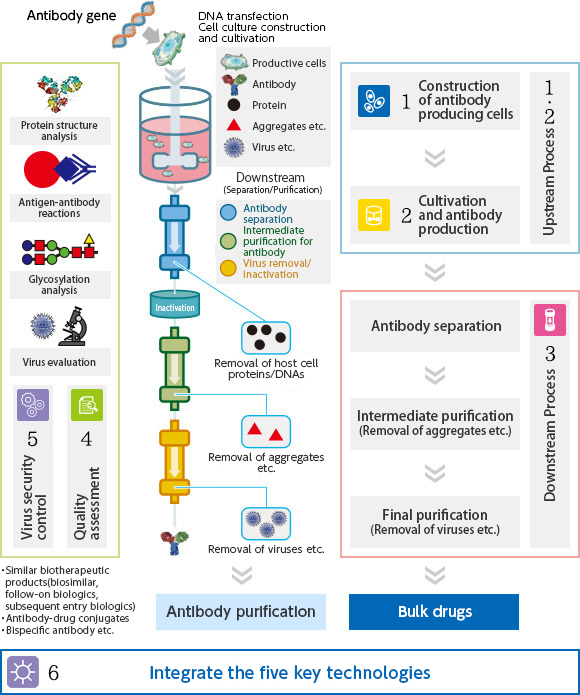
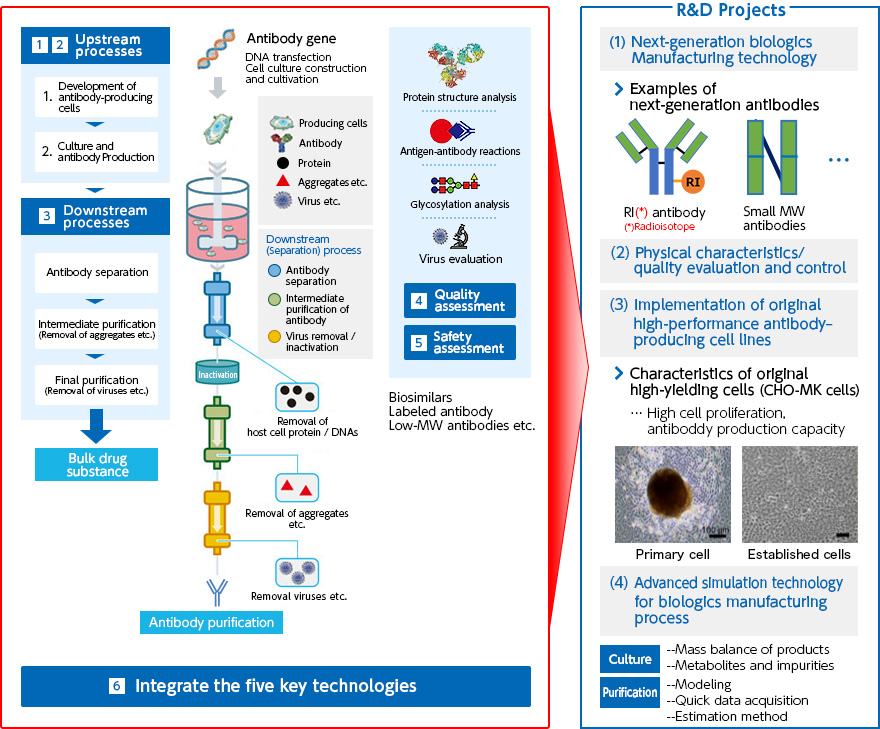
- -R&D subject 1:
- Technology for manufacturing next-generation therapeutic antibodies
- -R&D subject 2:
- Physical properties, quality evaluation and control methods for next-generation therapeutic antibodies
- -R&D subject 3:
- Implementation of original high-performance antibody-producing cell lines
- -R&D subject 4:
- Advanced simulation technology for biopharmaceutical manufacturing process
This project is being undertaken by MAB, which consists of Japanese companies, universities and public research institutions in biologics manufacturing.
Research Consortium
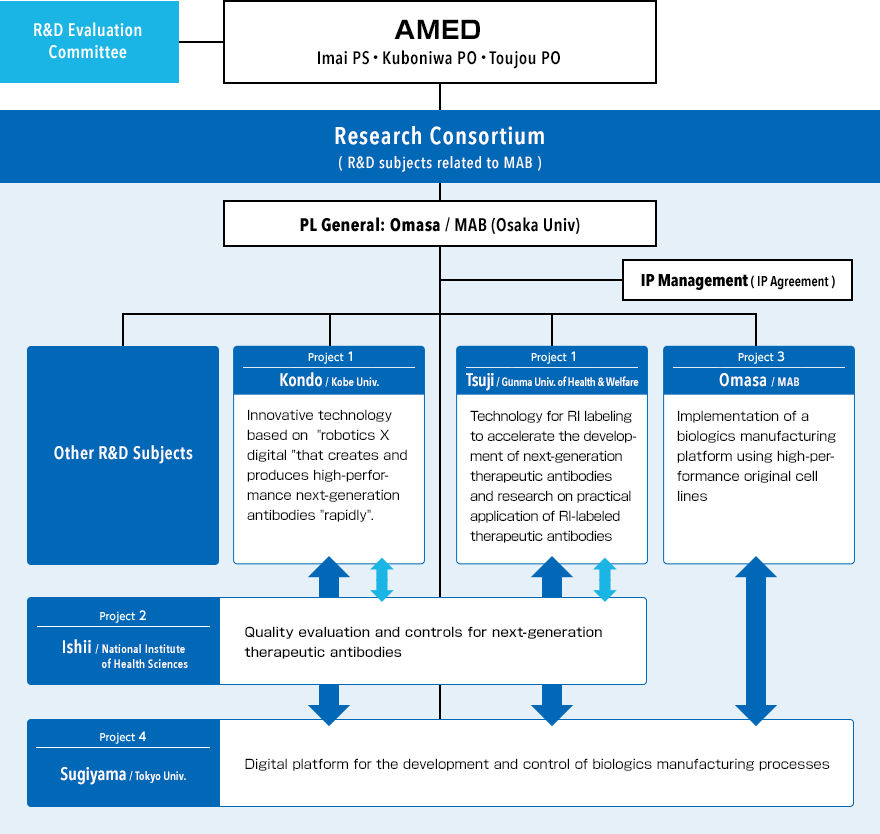
-FY2021~FY2025-
"Next-generation biologics manufacturing conforming to global standards"
 (October 01, 2024)
(October 01, 2024)
- *
- GMP : Abbreviation for Good Manufacturing Practice
Quality control standards for pharmaceutical products, etc., which are based on the Pharmaceutical Affairs Law of Japan, as well as the manufacturing quality control standards for pharmaceutical products, etc. established by the US Food and Drug Administration.
Kobe Consolidated Lab.

Osaka Consolidated Lab.

Yokohama Consolidated Lab.

Fukushima GMP Consolidated Lab.

High-quality Biologics Manufacturing Technology (FY2018-FY2020)
Establishment of advanced therapeutic antibodies manufacturing technology
R&D subjects
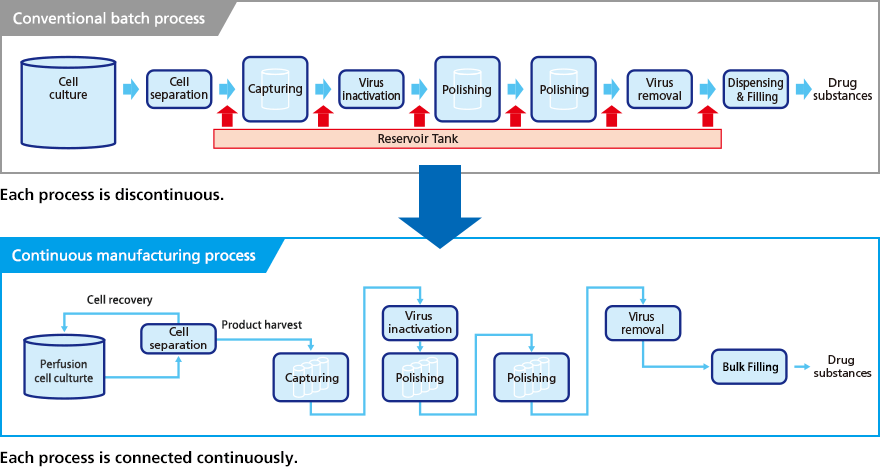
- Continuous production of therapeutic antibodies
- ◦Production cells
- ◦Quality control
- ◦Perfusion Cell culture
- ◦Virus safety management
- ◦PCC chromatography
- Consolidated manufacturing technology (Engineering run)
- Construction of continuous manufacturing process platform
- ◦Integrated type (Complete continuous manufacturing line)
- ◦Hybrid type (Combination batch process)
Assessing the large scale manufacturability of biopharmaceutical seeds
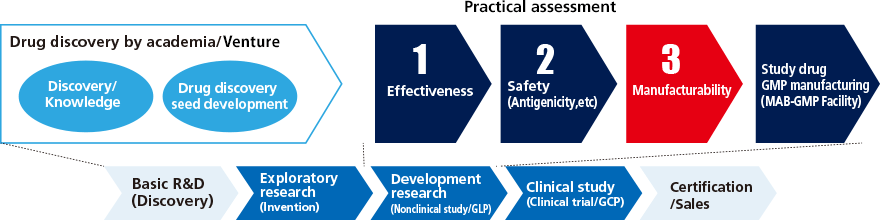
Manufacturing technology for next-generation antibody drugs that meet International standards
(FY2013-FY2017)
Development of productive cell line construction technology
- Rapid construction method of host cells
- Advanced and technology for original mammalian host cell improvement
- Platform development for production cell line establish by combining developed technologies
Development of high-performance cell culture technology
- Next-generation single-use components
- Advanced sensing and cell culture control technology
Development of advanced downstream technology
- High-performance chromatography media
- Single-use components for downstream processes
- Virus-removal and inactivation processes
- Package development for downstream processing
Development of advanced quality evaluation
- Structural change of biologics
- Aggregate formation of biologics
- Post-translational modification of biologics
- Evaluation of technologies and combining them
Development of virus control technology
- Establishing a platform for virus testing
- High-sensitive detection technology for virus control
Establishment of next generation platform technology adopted with international standards.
- Platform process development based on a single-use system
- Seamless platform development from small-scale to large-scale
- Strategic promotion toward international standardization
- System arrangement for organizing cell bank construction